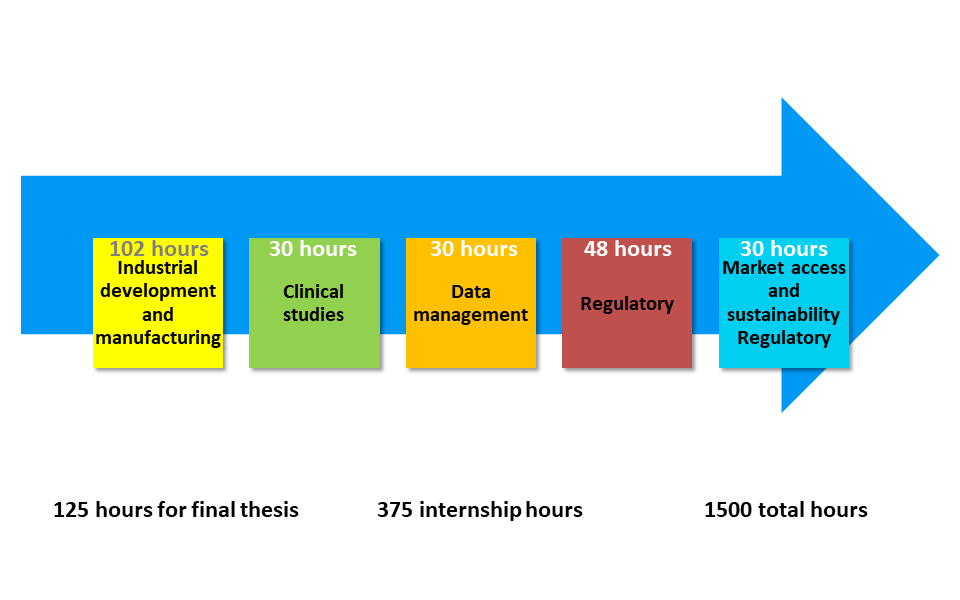
Our Post-graduate 2nd Level Master Programme is structured in 1500 hours, for a total of 60 training credits (CFU), of which:
- • 240 hours of face-to-face lectures;
- • 375 hours of internship;
- • 125 hours for the final test.
The remaining hours will be dedicated to individual study, workshops, discussion – individually and in groups – of leading cases, testimonials, and company visits, aimed at ensuring the full acquisition of conceptual tools and applications.
The second edition of the Post-graduate 2nd Level Master Programme will start in October. Classes will be held online, in live streaming, and will generally occur on Fridays (9.00 a.m. – 1.00 p.m. and 2.00 p.m. – 6.00 p.m.) and Saturdays (9.00 a.m. -1.00 p.m.). Once every 4-6 weeks students will be invited to join a class that will take place in Modena in-person and simultaneously online.
TEACHING MODULES & STAFF
Our Post-graduate 2nd Level Master Programme develops the following topics:
1) Industrial development and manufacturing;
2) Clinical studies of biopharmaceutical products and new concepts in trial design and statistical approaches;
3) Data management in the various industrial processes and exploitation;
4) Regulatory aspects for the market authorizations;
5) Market access and sustainability.
The teaching staff is composed of professors, researchers from private and public institutions, corporate directors, personnel managers, and qualified experts.
375 HOURS OF INTERNSHIP: WHEN AND WHERE
Internships will begin once lectures are over and will take place during weekdays, following the company’s working hours.
The internship period can be carried out at some selected companies among the list of our partners. Internship positions are available in Italy as well as in the other Countries of the collaborating partners.
The website will soon make public the list of the available partners for accepting students for internships. Such a list will be constantly updated as partners offer new positions. Please note that some partners may require a specific profile to accept candidates.